Mechanism of Serious Genetic Skin Disorder Uncovered
Professor Xu Tan’s research group of School of Pharmaceutical Sciences of Tsinghua University, in collaboration with Professor Yong Yang and Zhimiao Lin’s group of Peking University First Hospital, have identified a new causative gene and a novel disease causing mechanism of a serious genetic skin disorder-epidermolysis bullosa. This gene, named KLHL24, has been identified as an important gene to regulate the differentiation and protein homeostasis of the skin. The study has also uncovered a signaling transduction pathway for the ubiquitination and degradation of keratin proteins in the skin and suggested a new approach to treat related skin disorders.
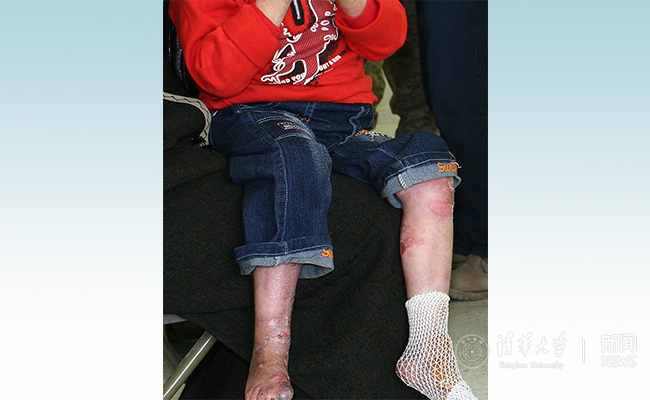
Figure 1. Picture of an EB patient (adapted from Wikipedia).
Epidermolysis bullosa (EB) is a serious genetic skin fragility disorder due to defects in structural proteins in the skin. Trivial trauma and frictions can cause blister formations and damages in the epidermis. Repeated skin damages lead to complications such as secondary infections and vision loss due to conjunctival scarring. EB patients suffer from skin fragility right from their birth, so they are called “Butterfly Babies” as their skin is as fragile as butterfly wings. EB has a high disability and mortality rate and is considered as the most serious genetic skin disorder and one of the most painful human genetic diseases. There is currently no safe and effective cure for EB.
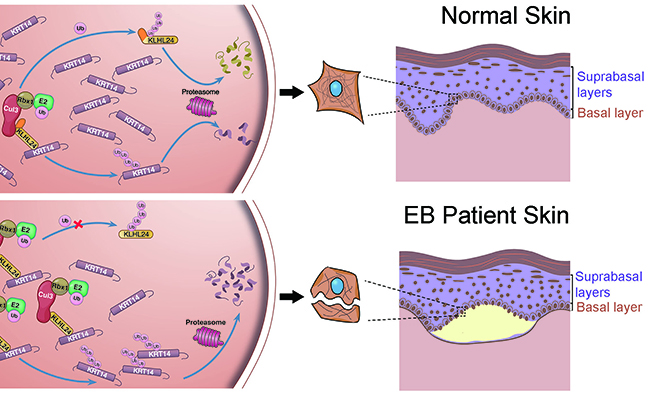
Figure 2. Schematic of the disease-causing mechanism of KLHL24 mutations. Upper panel: under normal condition, KLHL24 protein is tightly controlled by autoubiquitination and turnover of KRT14 by CUL3–RBX1–KLHL24 is at a low level. Lower panel: in the EBS cases we found, KLHL24is stabilized through abolished autoubiquitination. Overstabilized mutant KLHL24 leads to excessive ubiquitination and degradation of KRT14. The amount of KRT14 in basal keratinocytes is remarkably reduced, and the mechanical integrity of basal keratinocytes is compromised.
The clinical research from the Peking University First Hospital pinpointed a new type of EB that is caused by the mutation in the start codon of a gene encoding an enzyme called KLHL24, which is a ubiquitin ligase, an enzyme responsible for selecting proteins for degradation. The mutation in the start codon of the gene cause the translation of the protein starting from the 29th codon, thereby generating a protein missing the first 28 amino acids. Xu Tan’s group found that the shortened protein becomes more stable than its wild type counterpart due to abolished auto-ubiquitination. Further research identified the substrate protein of KLHL24 is a keratin protein – keratin 14. The stabilization of KLHL24 leads to excessive ubiquitination and degration of keratin 14. Since keratin 14 is an important structural protein for maintaining the integrity of the epidermis, the excessive degradation of keratin 14 will significantly increase the fragility of the skin and cause EB (Figure 2). The new research also identified KLHL24 as an important gene for regulating epidermis differentiation. In general, the study opened a new research area of the relationship between skin disease and keratin ubiquitination and suggests a new approach to treating skin disorders related to dysfunctional keratins. International peer reviewers commented that this study provides one of the most exciting results in the field and will have a far-reaching effect. On October 31st, the results were published as an Article in the top international genetics journal Nature Genetics, titled “Stabilizing mutations of KLHL24 ubiquitin ligase cause loss of keratin 14 and human skin fragility”. (Link: http://dx.doi.org/10.1038/ng.3701). The corresponding authors of the article are Xu Tan and Yong Yang. Shuo Li, a graduate student from the School of Life Sciences of Tsinghua and Zhimiao Lin and Cheng Feng from Peking University First Hospital are the co-first authors of the article. Professor Haiteng Deng of School of Life Sciences of Tsinghua and Professor Ting Chen from the National Institute of Biological Sciences have also contributed to the study. The study was funded by the National Nature Science Foundation and the Tsinghua-Peking Center for Life Sciences.

